A 3 year-old neutered male domestic shorthaired cat was rolled out of an OR after the chylothorax surgery (cysterna chyli ablation, pericardiectomy, and thoracic duct ligation) as well as the surgical correction of congenital peritoneopericardial diaphragmatic hernia (PPDH). An extensive pleural fibrosis was noted during surgery due to the suspected chronicity of the chylous effusion. A unilateral small-bore chest tube was placed into one of the hemithoraces at the conclusion of the surgical procedure. The intraoperative anesthesia monitoring was complicated by the inability to obtain indirect blood pressure measurements during the second half of the procedure despite the presence of otherwise stable monitoring parameters including end-tidal CO2. No significant blood loss was noted during the surgery.
Upon arrival to the ICU, the cat’s blood pressure was unobtainable, femoral pulses were weak, and mucous membranes were pale. The respiratory effort appeared normal and the respiratory rate was 50 breaths per minute. The fentanyl CRI was discontinued due to the prolong recovery and stuporous mentation. The point-of-care focused ultrasound showed hyperdynamic heart, mild-to-moderate amount of pleural effusion in the hemithorax without the drain, and the absence of abdominal fluid.
The venous blood gas showed the following results:
- pH = 7.09
- pCO2 = 73.4 mmHg
- HCO3- = 22.6 mmol/l
- BE = -7.4 mmol/l
- Lactate = 6.5 mmol/l
- PCV/TS = 40%/4.2 g/dl
The cat received two boluses of a crystalloid solution and started on a norepinephrine CRI due to the lack of response to fluids alone. At the same time, the pre-placed chest tube was aspirated and a new chest tube was placed on the opposite side. Eighty millilitres of pleural effusion were removed from the both sides and the repeat venous blood gas revealed the following results:
- pH = 7.06
- pCO2 = 77.9 mmHg
- HCO3 = 22.4 mmol/l
- BE = -8.1
- Lactate = 6.8 mmol/l
- PCV/TS = 39%/4.1 g/dl
The attempt to obtain an arterial blood gas sample from the femoral artery was unsuccessful due to very poor pulse quality. The cat’s breathing pattern subjectively remained within normal limits without evidence of dyspnea or superficial breathing.
A possibility to initiate positive pressure ventilation due to persistent severe hypercapnia was discussed with the owner, but was declined. The norepinephrine titrated up to 2 mcg/kg/min and a dose of hydrocortisone failed to correct a profound global hypoperfusion.
Case Discussion
This case presented a few challenges to the critical care team. Severe persistent hypercapnia after thoracotomy procedure will be a sign of profound hypoventilation in 9 out of 10 patients (see rearranged alveolar ventilation equation below):
- PACO2 = VCO2/VA
- PACO2 – partial pressure of carbon dioxide in the alveoli
- VCO2 – CO2 production
- VA – alveolar ventilation
In patients with constant CO2 production and relatively normal lung perfusion, there is an inverse relationship between PACO2 level and alveolar ventilation. Arterial, venous and end-tidal CO2 (PACO2) have a linear relationship between each other with an absolute difference around 2-5 mmHg in health (ETCO2 < PaCO2 < PvCO2).
But what are the relationships between them in animals experiencing profound circulatory shock?
What is the utility of the venous CO2 in this population of patients? Can it be used for assessment of a patient’s ventilatory status in this scenario?
The finding of arterial alkalemia and hypocapnea with mixed venous acidemia and hypercapnea has been reported in shock states (Mathias et al, 1988; Silva Jr. et al, 2011; Williams et al, 2014). Grundler et al. (1986). Using an anesthetized ventilator-dependent porcine preparation exposed to cardiac arrest, demonstrated a marked venous acidemia and hypercapnea (PvCO2, from 45.2 to 54.2 mmHg; pH, 7.41 to 7.31) in the presence of arterial alkalemia and hypocapnea (PaCO2, from 37.4 to 20.1 mmHg; pH, 7.46 to 7.54) after successful resuscitation. Paluch et al. (1985), using an anesthetized, mechanically ventilated canine model of endotoxemia, found a progressive increase in mixed venous PvCO2 (from 41 to 66 mmHg) with a relatively constant arterial PaCO2 (35 to 45 mmHg) 3 h after injection of endotoxin. Two studies of canine hemorrhagic shock (Halmagyi et al, 1970; Benjamin et al, 1986), using anesthetized ventilated dogs, have shown severe venous hypercapnea with reduced or normal arterial PaCO2 levels. This paradox was felt to result from a decreased clearance of carbon dioxide from the lungs when pulmonary blood flow was reduced. That is one of the reasons why we rely on ETCO2 during cardiopulmonary resuscitation as a surrogate of global and pulmonary perfusion.
An interesting experimental study was performed by Mathias and colleagues in 1988 where researches decided to look at the disparity between arterial and venous blood gases in spontaneously breathing dogs with shock. In a chronically instrumented unanesthetized canine model of acute cardiac tamponade breathing room air, they studied the effect of a graded decline in cardiac output on arterial and mixed venous pH, PCO2, and PO2. Cardiac tamponade resulted in a profound arterial respiratory alkalosis, whereas mixed venous pH, PCO2, and calculated serum bicarbonate levels remained relatively unchanged. As intrapericardial pressure increased and cardiac output declined, the difference between arterial and mixed venous PCO2 progressively increased (see the graph from the study below).
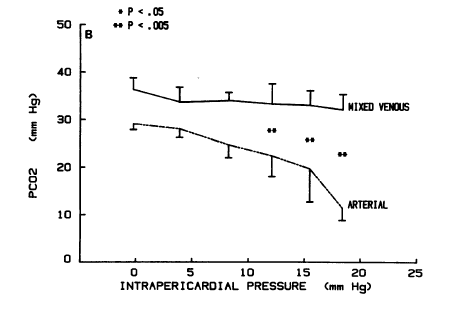
In other words, both types of experiments (mechanically ventilated vs spontaneously breathing animals in shock) showed a clinically significant increase in gradient of arterial and venous CO2, however a spontaneously breathing model of shock did not reveal the profound venous hypercapnea that was noted in the mechanically ventilated animals in shock. As I mentioned before, the elevated arterial-venous gradient occurs due to decreased CO2 delivery to the lungs when pulmonary blood flow is reduced. Spontaneously breathing patients are able to maintain normal venous PCO2 by increasing their minute ventilation that was demonstrated in the Mathias et al. study. However, the fixed minute ventilation in mechanically ventilated animals prevented them from hyperventilation and ability to maintain venous CO2 at a steady state leading to severe venous hypercapnea.
Unfortunately, we were not able to obtain an arterial blood sample for direct comparison with venous blood in our cat. It is likely that the cat’s reduced vital capacity of the lungs compromised by the restrictive pleural fibrosis resulted in a situation similar to the experimental model of hemorrhagic shock in animals undergoing mechanical ventilation (i.e. fixed minute ventilation). We can speculate that the cat might have not been able to compensate for the increased need to elevate minute ventilation in order to blow out excessive CO2 produced in the tissues. This lack of compensation was caused by the fixed minute ventilation due to his restrictive lung disease. This may also explain why the placement of the second chest tube and the complete evacuation of the pleural effusion did not resolve the problem of venous hypercapnea.
Another venous blood gas finding that is worth mentioning is the discrepancy between HCO3- and BE, where HCO3- was within normal limits at 22.6 mmol/l and BE was moderately negative at -8 mmol/l. This is a classic example of a situation when BE gives you more objective information about the metabolic component. As you remember, bicarbonate is a calculated value derived from blood pH and pCO2 via the Henderson-Hasselbalch equation incorporated in the machine algorithm. Extreme changes in pCO2 (severe hypocapnea or hypercapnea) will lead to significant changes in HCO3- value. Base excess is defined as the amount of strong acid or base that must be added to each liter of fully oxygenated blood to return the pH to 7.40 at a temperature of 37°C and a pCO2 of 40 mmHg. Hence, it will not be influenced by pCO2 derangements and is going to be more accurate in assessment of metabolic component of acid-base status in these patients as opposed to bicarbonate.
The bottom line is that we should be careful interpreting venous CO2 levels in patients with severe shock. Venous hypercapnea in these scenarios may be a consequence of profound hypoperfusion that confounds interpretation of a patient’s ventilatory status.
References
- Mathias et al. Mixed venous blood gases are superior to arterial blood gases in assessing acid-base status and oxygenation during acute cardiac tamponade in dogs. J Clin Invest, 1988.
- Silva Jr. et al. A large Venous-Arterial PCO(2) Is Associated with Poor Outcomes in Surgical Patients. Anesthesiol Res Pract, 2011.
- Williams et al. Arterial vs venous blood gas differences during hemorrhagic shock. World J Crit Care Med, 2014.
- Grundler et al. Arteriovenous carbon dioxide and pH gradients during cardiac arrest. Circulation, 1986.
- Paluch et al. Regional CO2 production in canine endotoxemia. Anesthesiology, 1985.
- Halmagyi et al. Hidden hypercapnia in hemorrhagic hypotension. Anesthesiology, 1970.
- Benjamin et al. Venous hypercarbia in canine hemorrhagic shock. Crit Care Med, 1986.


Maybe I’m confusing something ..
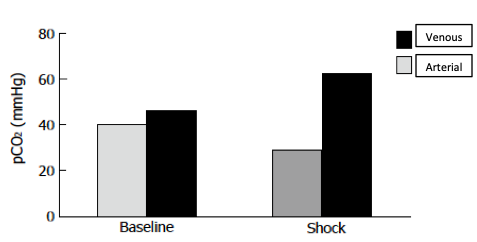
Why in the scheme by “Williams et al, 2014” on the contrary, hypocapnia in vein and hypercapnia in artery in conditions of shock are indicated. (The study also included rabbits, not dogs.)
Hi there! This is an excellent question and a great catch. It turns out that the authors of this paper mislabeled the columns (arterial vs. venous). The description of the figure and the actual figure do not match each other. Here is the excerpt from the paper: “In comparing pCO2 at baseline and shock, a non-significant decrease was observed in arterial pCO2 (40.0 ± 9.1 mmHg vs 28.9 ± 7.1 mmHg, P > 0.05), while venous blood samples demonstrated a non-significant trend towards increased pCO2 (46.0 ± 10.1 mmHg vs 62.8 ± 15.3 mmHg, P > 0.05), as shown in Figure 1C”. Therefore, the grey column is supposed to be arterial blood, whereas the black column is meant to be venous blood. And you are absolutely right that they were rabbits and not dogs.
I am going to go ahead and switch the labels to avoid confusion. Thank you very much for your input.
Cheers,
Igor
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Your article helped me a lot, is there any more related content? Thanks!
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.