This blog post commences a series of articles on diagnostic and therapeutic approaches to potassium and sodium derangements. This is meant to be a quick reference/guideline for emergency veterinarians, students and technicians. All readers are welcome to leave feedback and comments below.
Diagnosis of hyperkalemia
All causes of hyperkalemia may be divided into 3 big groups:
- Increased intake
- Translocation of K+ from intracellular fluid (ICF) to extracellular (ECF)
- Reduced excretion

Step-by-step approach to diagnosis
Step 1: Consider pseudohyperkalemia
- Platelet count > 1 million/uL
- Hemolysis (especially, in Japanese breeds such as Akita, Shiba, and Sharpei)
Step 2: Review current medications that may lead to hyperkalemia
- K+ containing IV fluids;
- Thrombolytic therapy (leading to reperfusion and release of potassium from the intracellular space);
- Cancer chemotherapy (leading to acute tumor lysis syndrome);
- ACE inhibitors and angiotensin II (AII) receptor blockers (e.g. telmisartan): result in decreased production of aldosterone by the adrenal glands and the lack of AII blunts glomerular efferent arteriolar constriction, which potentially can decrease delivery of sodium and water to the distal nephron (due to reduced GFR) and impair renal potassium excretion);
- K+ sparing diuretics (e.g. spironolactone);
- B-blockers;
- Cardiac glycosides (inhibit Na/K-ATPase and can cause hyperkalemia and arrhythmias);
- Lysine or arginine infusion (these amino acids may enter cells in exchange for potassium);
- NSAIDs (inhibit prostaglandins that stimulate renin release; downregulation of RAAS leading to hyperkalemia);
- Heparin (impairs aldosterone production by decreasing the number and affinity of angiotensin II receptors in the zona glomerulosa of the adrenal glands and may contribute to hyperkalemia in the presence of other predisposing factors);
- Trimethoprim;
- Cyclosporin A, tacrolimus (decreased aldosterone production, inhibition of Na, K-ATPase, and interference with luminal potassium channels in renal tubular cells).
Step 3: Rule out DKA (history, hyperglycemia, ketonuria, acid-base status)
- Even though DKA patients usually have whole body K+ depletion, they may present with hyperkalemia due to insulin insufficiency and osmotic drag of K+ into extracellular space
Step 4: Is the patient azotemic?
- Reduced renal excretion of K+ is the most common cause of hyperK+;
- Palpate the bladder to rule out urethral obstruction; ultrasound renal pelvises to rule out hydronephrosis secondary to ureteral obstruction;
- Assess for free peritoneal or retroperitoneal fluid on POCUS (point-of-care ultrasound); if present, sample it and compare fluid creatinine and K+ with serum creatinine and K+;
- Assess volume status of the patient; if signs of global hypoperfusion are present, correct hypovolemia or other causes of shock, and recheck blood K+;
- Measure USG before fluid therapy to differentiate causes of azotemia;
- Rule out hypoadrenocorticism or Pseudoaddison’s (see step 5);
- If prerenal/postrenal/adrenal insufficiency causes of azotemia are ruled out; the patient is likely having intrinsic renal disease;
Step 5: Consider a possibility of hypoadrenocorticism or Pseudoaddison’s (hypoadrenocorticism-like syndrome)
- If you have a high index of suspicion, perform an ACTH stimulation test; if the index of suspicion is low, submit a basal cortisol (basal cortisol >2 ug/dL will rule out Addison’s, but in dogs with basal cortisol <2 ug/dL, an ACTH stim test should be performed) – Bovens et al. 2014;
- Pseudoaddison’s may be suspected in patients with GI losses (especially due to trichuriasis, salmonellosis), pleural or peritoneal effusions (usually chronic), late pregnancy;
- The hyperkalemia observed in these situations is thought to arise from decreased renal excretion of potassium as a consequence of volume depletion (e.g., gastrointestinal fluid loss and third-space loss of fluid) and decreased distal renal tubular flow.
Step 6: Assess acid-base status
- Acute metabolic acidosis may lead to ICF –> ECF translocation of K+ in exchange of H+.
Treatment of hyperkalemia
- Obtain ECG (especially, if K+ >6 mmol/l)
- Both bradyarrhythmias and tachyarrhythmias may occur;
- Classic changes that are not always present: “tenting” of T waves, shortening of QT (due to abnormally rapid repolarization), prolongation of PR-interval, widening of QRS (slow conduction via AV system), decreased amplitude and widening of the P wave, absence of P waves, a sinoventricular rhythm (see the post published by Laura Cole on wide-complex tachycardia);
- If ECG abnormalities are present and/or patient is hemodynamically unstable due to abnormal heart rate and/or rhythm, administer Ca gluconate 10% 0.5-1 ml/kg IV over 10-30 min with constant ECG monitoring;
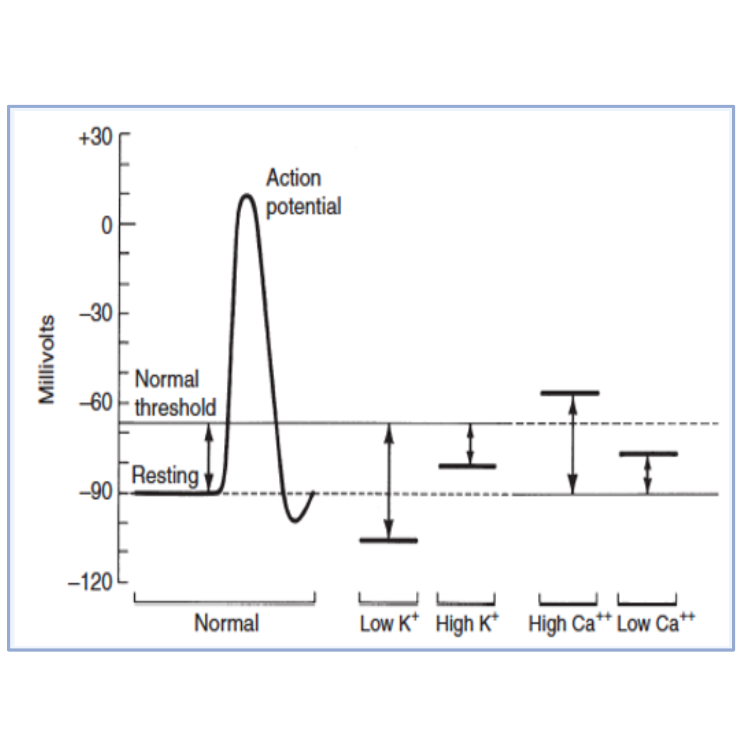
- This treatment will not reduce plasma K+, but will decrease excitability of myocardium via effects on threshold potential (it will become more positive resulting in increased distance between resting membrane potential and threshold membrane potential – see Figure 1);
- Bradycardia may signal the onset of cardiotoxicity arising from excessively rapid infusion of calcium +/- sudden elevation of the ST segment or shortening of the QT–interval
- Simultaneously, try to identify the underlying cause of hyperkalemia (see step-by-step approach to diagnosis above) and address it as soon as possible;
- Consider IV fluids if they are not contraindicated;
- Potassium-deficient balanced crystalloids (LRS, Norm-R) are as efficient as K-free solutions (NaCl 0.9%) (Drobatz et al. 2008) because they don’t worsen metabolic acidosis caused by high chloride content (e.g. in NaCl 0.9%) resulting in ICF –> ECF translocation of K+;
- Discontinue all drugs that promote hyperkalemia (see the list above)
- Consider administration of medications that will promote intracellular shift of K+ that will buy some time while addressing the underlying condition
- Dextrose 50% – give 0.5-1 ml/kg diluted 1:3 with NaCl 0.9% or balanced crystalloid. Dextrose administration will induce endogenous insulin release that will shift K+ intracellular;
- 5% dextrose CRI may be considered if prolonged effect is desired;
- Combination of dextrose and regular insulin: this combination will be more efficient than dextrose alone, however it will require close monitoring of blood glucose during the next few hours;
- General recommendation is 0.1-0.2 U/kg of regular insulin for every 1 ml/kg of 50% dextrose
- Measure BG in 15-30 min after insulin/dextrose administration and then every 30-60 min for the next 2-4 hours as needed.
- Terbutaline (B-agonist) – give 0.01 mg/kg IM or SQ once; some sources allow IV administration, but in the author’s experience, this may lead to higher risks of tachycardia/tachyarrhythmia (thus, IV administration should be considered only during CPR/near-CPR situations);
- If a patient is suspected to have an intrinsic renal disease, medications that promote intracellular translocation of K+ may have only transient effect while renal excretion is significantly diminished;
- If initial therapy to optimize renal function did not improve K+ renal excretion and the patient has moderate to severe hyperK+, hemodialysis (or peritoneal dialysis) should be initiated;
- You may consider administration of loop diuretics (furosemide) only in patients with refractory hyperkalemia that are euvolemic or hypervolemic, and they don’t have contraindications to diuretic therapy – see the post published by Laura Cole on the use of furosemide in AKI patients;
Supplemental information
Platelets and K+
- Serum potassium concentrations exceed plasma concentrations because potassium is released from platelets during the clotting process;
- There is a positive correlation between platelet count and serum potassium concentration in dogs. The difference between serum and plasma potassium concentrations is most pronounced in animals with thrombocytosis;
- In one study, serum potassium concentration was greater than plasma potassium concentration by a mean of 0.63 mEq/L in dogs with normal platelet counts and by a mean of 1.55 mEq/L in dogs with thrombocytosis (Reimann et al. 1989).
RBCs and K+
- The potassium content of erythrocytes varies in mammalian species, and hemolysis can result in hyperkalemia in species that have high red cell potassium concentrations;
- Normal adult canine and feline red cells usually contain potassium in concentrations similar to those of plasma, and hemolysis is not associated with hyperkalemia;
- In one study, storage of canine red cells in citrate-phosphate-dextrose- adenine for 40 days resulted in an increase in plasma potassium concentration from 5 to almost 9 mEq/L (Price et al. 1988);
- The potassium concentrations of red cells from neonatal dogs are higher than those of red cells from adult dogs; in one study, mean red cell potassium concentrations in puppies were 19.0 mEq/L at 1 day of age, 15.1 mEq/L at 5 weeks of age, and 8.7 mEq/L at 13 weeks of age (Coulter et al. 1972);
- In adult Akitas, red cell potassium concentrations may exceed 70 mEq/L, and hemolysis results in a progressive increase in plasma potassium concentration (up to 24 mEq/L) during storage of blood;
- Dogs may be divided genetically into two groups based on the presence or absence of Na , K-ATPase activity in the membranes of their mature red cells; dogs with red cell membrane Na, K-ATPase activity maintain high intracellular potassium concentrations, whereas those without red cell Na, K-ATPase activity maintain red cell potassium concentrations similar to those of plasma;
- The HK phenotype is inherited as an autosomal recessive trait and occurs with an incidence of 26% to 38% in the Shiba and Akita breeds in Japan and 42% in the Jindo breed in Korea (Fujise et al. 1997);
- The HK pheno- type also may be seen in the Chinese shar-pei breed and can cause pseudohyperkalemia;
- Pseudohyperkalemia also has been reported in a dog with acute lymphoblastic leukemia before chemotherapy;
- Use of plasma from small blood samples collected in an excessive volume of tripotassium ethylenediaminetetraacetic acid also may result in measured hyperkalemia.
References
- Reimann KA, Knowlen GG, Tvedten HW. Factitious hyperkalemia in dogs with thrombocytosis: the effect of platelets on serum potassium concentration. J Vet Intern Med 1989;3:47;
- Price GS, Armstrong PJ, McLeod DA, et al. Evaluation of citrate-phosphate-dextrose-adenine as a storage medium for packed canine erythrocytes. J Vet Intern Med 1988;2:126;
- Coulter DB, Small LL. Sodium and potassium concentrations of erythrocytes from perinatal, immature, and adult dogs. Cornell Vet 1972;63:462.
- Fujise H, Higa K, Nakayama T, et al. Incidence of dogs possessing red blood cells with high K in Japan and East Asia. J Vet Med Sci 1997;59:495;
- Drobatz et al. The influence of crystalloid type on acid–base and electrolyte status of cats with urethral obstruction. JVECC 2008;
- Bovens et al. Basal Serum Cortisol Concentration as a Screening Test for Hypoadrenocorticism in Dogs. JVIM 2014.

Hi Igor
Thanks for a nice practical guide on how to approach hyperkalaemia.
Do you ever use bicarbonate in management of hyperkalaemia with co-exisiting academia? Anecdotally I find bicarbonate to be more effective in stabilising the hyperkalaemia for longer that insulin/glucose in academic cats with bilateral ureteral obstruction that come in overnight and are in holding pattern until can expedite to surgery in AM. I know that bicarbonate in people has not been shown to be particularly effective in management of hyperkalaemia but my experience is different and was interested in your thoughts. Interestingly, a recent Lancet study looking at bicarbonate use in ICU showed decrease mortality with AKI patients with academia:
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31080-8/fulltext.. But perhaps a detailed chat at pros and cons of bicarbonate per see is a topic for another day!
Thanks
Laura
Hi Laura! Thank you for your question. I, personally, have a minimal experience in using bicarb in animals with hyperkalemia, but I am not opposed to it. I agree that those patients with persistent hyperkalemia due to a poor excretory renal function that need either a surgical procedure to relieve an obstruction or hemodialysis to replace their renal function are very challenging and sometimes you need to do everything you can to bridge them to the definitive care the following day. I think bicarbonate administration is especially appropriate in patients with hyperchloremic metabolic acidosis (i.e. secondary to external losses of bicarb and not due to elevated anion gap) such as patients with a renal disease combined with RTA because you are just replacing what they’ve lost. To my knowledge, human intensivists do that quite frequently, but they never give it rapidly, only as a slow infusion to avoid common complications related to bicarb administration.
That being said, in my last two patients that I remember who had very persistent hyperkalemia (one dog needed hemodialysis and was oligoanuric; another cat had bilateral ureteral obstruction), I was able to manage them with dextrose CRI + intermittent boluses of terbutaline and insulin. With these treatments I kept their K+ below 6-6.5, but, maybe in retrospect, the bicarb therapy would have increased the efficiency of this treatment and allowed me to avoid frequent BG monitoring.