Furosemide is the most commonly used diuretic in critical care and is frequently used in the management of acute kidney injury (AKI). However, the benefits of furosemide administration in AKI has long been questioned and there are concerns over the possible harmful effects of furosemide including diuretic-induced AKI. To further evaluate the role of furosemide in management of AKI, let us consider a case.
A 3 year male neutered 15 kg Springer Spaniel presents to your hospital having been diagnosed with AKI. Initial blood work revealed severe azotemia (creatinine 7.92mg/dL [700 μmol/L], BUN 98 mg/dL [35 mmol/L]) and borderline hyperkalaemia (4.2 mmol/L). After 12 hours of fluid therapy at 4 ml/kg/hr repeat blood work reveals a worsening azotemia (creatinine 9.05mg/dL [800μmol/L]) and hyperkalemia 6 mmol/L. Urine output is 0.1 ml/kg/hr. The dog now weighs 17.25 kg, has chemosis, pleural and peritoneal fluid. Do you give furosemide, or do you recommend immediate hemodialysis?
You decide to give a single dose of 0.25 mg/kg furosemide and there was no further urine production over the next 2 hours. You then give a 1 mg/kg bolus and the dog produces 6 ml/kg/hr of urine. Over the next 12 hours the UOP increases to 10 ml/kg/hr and the creatinine has increased to 9.61mg/dL [850μmol/L]. What does this mean?
To answer these questions, we need to understand the pharmacology of furosemide.
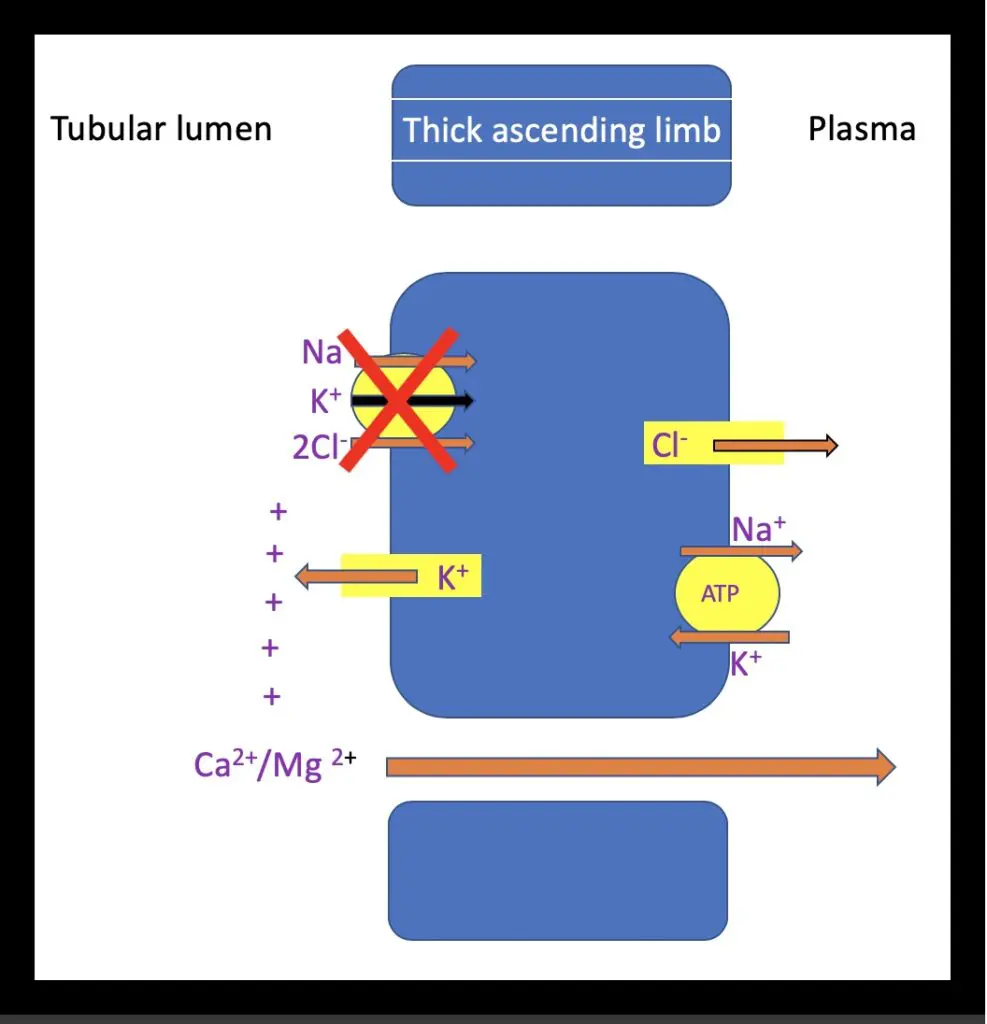
Furosemide exerts its action by exclusively inhibiting the Na/K/2Cl (NKCC2) co-transporter on the luminal membrane of the thick ascending loop of Henle. In plasma, 98% of furosemide is protein bound. To reach the site of action protein bound furosemide is secreted from the plasma in to the lumen of the proximal tubules via an organic anion transporter (OAT1), where it is then delivered to its site of action in the thick ascending limb. The inhibition of this transporter and subsequent decrease in sodium and chloride resorption results in natriuresis and diuresis, dissipating the medullary osmotic gradient and concurrently causing a reduction in calcium and magnesium reabsorption (Figure 2). Increased distal delivery of sodium leads to sodium potassium exchange promoting kaliuresis. Na/K/CL transporters are not confined to the kidney, NKCC1 transporters are present in organs including the vasculature, respiratory tract and ear.
Back to the case
Despite fluid therapy the dog has a worsening azotemia, hyperkalemia and is oliguric. It also has signs of fluid overload demonstrated by a 15% weight gain, chemosis, pleural and peritoneal fluid (Claure-Del Granado & Mehta 2016). Fluid overload, oligo/anuria and hyperkalemia are all indicators for CRRT (Bellomo et al, 2009).
The question is should furosemide be administered initially, in place of hemodialysis?
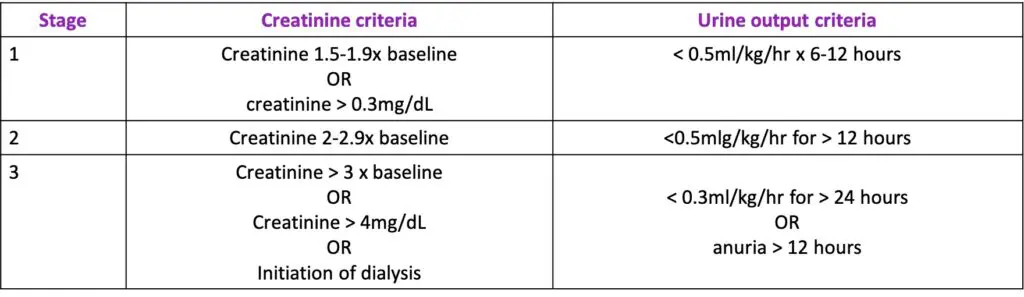
The benefit of furosemide in management of AKI has long been questioned. Diuretics have been found to be a risk factor for AKI (Levi et al, 2012) and various studies have shown furosemide to either exhibit neutral or deleterious effects in AKI treatment (Gram et al 2011, Mehta et al 2002, Krzych et 2019). One such observational study looking at diuretic use in AKI (diagnosed by elevated serum creatinine only) found diuretic use to be associated with an increased risk of death and non-recovery of renal function (Mehta et al 2002). A more recent study using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria for diagnosis of AKI (figure 3) showed furosemide administration to be associated with improved short-term survival and recovery of renal function in critically ill patients with AKI, particularly those with AKI UO stage 2-3 (Zhao et al 2020). Some studies suggest the use of furosemide in AKI can lead to a delay in the initiation of renal replacement therapy (RRT) and early RRT itself has been associated with improved recovery of renal function but also with increased risk of adverse events (Fayd et al 2018, Zarbock et al 2016). However, a more recent publication showed furosemide not to have a significant effect on the requirement for RRT or outcome (Krzych et al 2019).
AKI is commonly reported as an adverse effect of diuretic use and a worsening azotemia is not uncommonly seen post diuretic administration. However, this azotemia should not be automatically interpreted as true worsening of renal function. Whilst most creatinine undergoes glomerular filtration, some is actively secreted in to the proximal convoluted tubules via the same organic acid transporter as furosemide (OAT1). In patients with renal disease this secretion increases relative to glomerular filtration. Furosemide may compete with creatinine for this transporter and this can lead to a relative inconsequential increase in creatinine (Andreev et al 1999). Furthermore, creatinine is measured as a concentration in serum; an increase in serum creatinine concentration in combination with increase in hematocrit may purely indicate a reduction intravascular volume and effective decongestion; termed “pseudo-worsening renal function” rather than actual worsening of renal function (Bagshaw et al 2007).
Furosemide may compete with creatinine for the transporter (OAT1) and this can lead to a relative inconsequential increase in creatinine.
(Andreev et al, 1999)
Furosemide also has proposed beneficial effects in the management of AKI including resolution of intra-renal congestion via renal vascular dilation and decrease in energy expenditure and oxygen consumption of tubular cells via inactivation of metabolically active NKCC2 transporters (Ho et al 2010). Furosemide has a particularly important role in the management of fluid overload associated with AKI as promotion of diuresis can result in a reduction in renal venous congestion (Joannidis et al 2017).
Furosemide has the potential to lead to significant diuresis in patients with AKI but high doses of frusemide (1-2 mg/kg) may be required to ensure furosemide reaches its site of action in the presence of significant tubular dysfunction. However, repeated doses may lead to significant increases in side effects, particularly ototoxicity. Therefore, repeated furosemide administration in patients with fluid overload who are not diuretic-responsive is not recommended. Hemodialysis is usually recommended in these cases (Joannidis et al 2017).
The diuresis associated with furosemide treatment in AKI is not a direct indication of beneficial effect of furosemide on renal function, instead it is an indicator of functional tubular cells (Ho et al 2010). Furosemide administration may therefore be a useful tool to predict the severity of AKI, commonly termed a furosemide stress test. One human study has shown that UOP < 200mls at 2 hours post administration of 1-1.5 mg/kg furosemide intravenously was associated with an increased risk of renal associated death (Chawla et al 2013).
Again..Back to the case
Furosemide administration was chosen in this case over immediate hemodialysis.
The dog produced a large volume of urine post furosemide administration and, thus, may be considered “furosemide responsive.” This response to furosemide may indicate less severe tubular dysfunction but this is not synonymous with full renal recovery. This dog still remains at increased risk of longstanding renal injury and death. Post furosemide administration the dog became markedly polyuric and the azotemia worsened. This worsening azotemia secondary to successful diuresis, is not unexpected. However, careful monitoring of blood pressure, hydration status, including body weight is important alongside monitoring and matching urinary output in all AKI patients and one should be extra vigilant when diuretics have been used as part of the case management.
The Bottom Line
Furosemide use has a role in management of oligo/anuric AKI and associated fluid overload and may be a useful tool to determine disease severity. However, inappropriate use of furosemide in a hypovolemic and dehydrated patient could contribute to worsening of the AKI.
References
- Claure-Del Granado & Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrology2016; 17:109 DOI 10.1186/s12882-016-0323-6
- Bellomo R, Cass A, Cole L et al (2009) Intensity of continuous renal-replacemnet therapy in critically ill patients. N Engl J Med 2009;361:1627-1638. DOI: 10.1056/NEJMoa0902413
- Levi TM, Rocha MS, Almeida DN, et al. Furosemide is associated with acute kidney injury in critically ill patients. Brazilian Journal of Medical and Biology Research 2012;45:827–833.
- Grams ME, Estrella MM, Coresh J,et al. Fluid balance, diuretic use, and mortality in acute kidney injury. Clinical Journal of the American Society of Nephrology. 2011;6:966–73.
- Mehta RL, Pascual, Soroko S, et al. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. Journal of the American Medical Association 2002; 27: 288(20):2547-2553.
- Krzych LJ, Czempik PF. Impact of furosemide on mortality and the requirement for renal replacement therapy in acute kidney injury: A systematic review and meta-analysis of randomised trials. Annals of Intensive Care 2019;9:85. doi:10.1186/s13613-019-0557-0
- Zhao GJ, Xu C, Ying JC, et al. Association between furosemide administration and outcomes in critically ill patients with acute kidney injury. Critical Care 2020; 24: 75 DOI: https://doi.org/10.1186/s13054-020-2798-6.
- Fayad A, Buamscha DG, Ciapponi A. Timing of renal replacement therapy initiation for acute kidney injury. Cochrane Database Systematic Review 2018;4:10:CD010613
- Zarbock A, Kellum JA, Schmidt C, et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. Journal of the American Medical Association 2016; 315:2190-2199.
- Andreev E, Koopman E, Arisz L. A rise in plasma creatinine that is not a sign of renal failure: which drugs can be responsible? Journal of Internal Medicine 1999; 246(3):247-52.
- Bagshaw SM, Delaney A, Haase M, et al. Loop diuretics in the management of acute renal failure: a systematic review and meta-analysis. Critical Care and Resuscitation Journal 2007;9:60–68.
- Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010; 65:283–293
- Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Medicine 2017; 43:730–749.
- Chawla LS, Davison DL, Brasha-Mitchell E et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Critical Care 2013; 17:R207.




Awesome job on the article Laura!
Hi Laura! Great article!
I have two questions for you:
1. At RVC, do you guys routinely place urinary catheters to all patients with oligoanuric or polyuric AKI patients?
I have good experience with managing these patients without u-catheters by just q6h body weight checks, frequent exams and ultrasonographic bladder assessments. The concern is iatrogenic UTI that will exacerbate existing renal disease.
2. You mentioned the dose of furosemide 1-2 mg/kg being high in patients with AKI. Is it the highest dose you administered?
There are some human papers where they administered furosemide 3-6 mg/kg or even higher in patients with severe oligoanuric AKI because of a lack of response to lower doses since the secretion of furosemide across the proximal tubular wall is impaired in severe cases. Definitely, high repeated doses will significantly increase the likelihood of adverse reactions with ototoxicity being the most common, but I have experience of giving 4-8 mg/kg of furosemide as a single dose to patients with anuric AKI that are fluid overloaded to manage fluid balance until the dialysis is ready to go. If this dose did not work, I am 100% sure that this patient is furosemide-unresponsive and would initiate the dialysis as soon as possible without giving more furosemide.
Hi Igor
Thanks for the comments
RE Urine output and urinary catheter – I have definitely moved away from urinary catheter placement in ambulatory cases for the reasons you mentioned. We had had too many hospital acquired nasty UTI which is the last thing these patients need! And yes, despite bladder volume assessment reported to be less reliable at large and small sizes you can infer if there is any urine production between scans and with the polyuric ones the nurses are very much on in with catching urine, weighing into pads alongside weight checks.
RE frusemide dose I have only given max dose of 2mg/kg as bolus but have then have them on CRI 0.5-1mg/kg/hr whilst discussing next steps with owner and CRRT team. Would be interested to know how many of your cases that did not respond to 1-2mg/kg responded to 4-8mg/kg dose.
There weren’t many, just a few. I have not seen obvious side effects. Ultimately, if a patient doesn’t respond to 1-2 mg/kg dose of furosemide, its kidneys are in a very bad shape, and giving a higher furosemide dose will be my last-ditch effort if it is dying from fluid overload and RRT is not available for some reason right away.
Great article/case example, thanks Laura
There’s a review looking at the furosemide response test to predict AKI/need for RRT in human medicine that’s just been published including some of your references:
Chen et al Furosemide stress test as a predictive marker of acute kidney injury progression or renal replacement therapy: a systemic review and meta-analysis. Critical Care. 2020;24(202)
They pooled data from various trials and found furosemide response test was useful for predicting AKI progression but struggled to assess furosemide response as predictive for need for RRT, especially as it’s hard to say when its optimal to start RRT in the first place.
Thanks Nadine for your comment – Super interesting area. Just need data in our veterinary patients but anecdotally I think many of us agree if they don’t respond to frusemide things are a lot worse.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.