A 7 year-old female spayed Pembroke Welsh Corgi was presented to a veterinary teaching hospital for evaluation of acute onset generalized clonic-tonic seizures, obtunded mentation and petechiation. Her primary veterinarian previously saw the dog two days ago when she developed anorexia. Multiple petechiations were noted on her skin at that time. Her initial blood work revealed severe thrombocytopenia at 5-10 K/uL (RI, 190-450 K/uL). The rest of the work-up (thoracic/abdominal radiographs and tick-borne disease testing) was unremarkable, and she was prescribed prednisone for suspected primary immune-mediated thrombocytopenia (ITP). The following day, she developed two clonic-tonic generalized seizures that lasted 1-2 minutes each. Her mentation became progressively worse, and she had several seizures on the day of presentation to the teaching hospital.
On presentation, the patient appeared obtunded and had normal cranial nerve reflexes. The dog’s respiratory rate and effort were increased, and the rectal examination showed melena. A very low platelet count was confirmed by a blood smear evaluation. It was believed that the combination of the patient’s clinical signs could be explained by multifocal bleeding secondary to ITP (forebrain, lung parenchyma and gastrointestinal tract).
Soon after presentation, the dog was placed into the oxygen cage and was given levetiracetam intravenously at 60 mg/kg to prevent seizures and lyophilized platelet concentrate infusion due to the suspected multifocal life-threatening bleeding secondary to severe thrombocytopenia. An injection of vincristine was ordered as well.


Over the next hour, the patient’s mentation progressively worsened and she developed bilateral mydriasis with unresponsive pupils. As the mentation was worsening from obtundation to stupor, the bolus of 7.2% hypertonic saline was given at a dose of 3 ml/kg IV, however no improvement in mentation was noted. Two hours later, the dog became comatose and brain herniation was suspected.
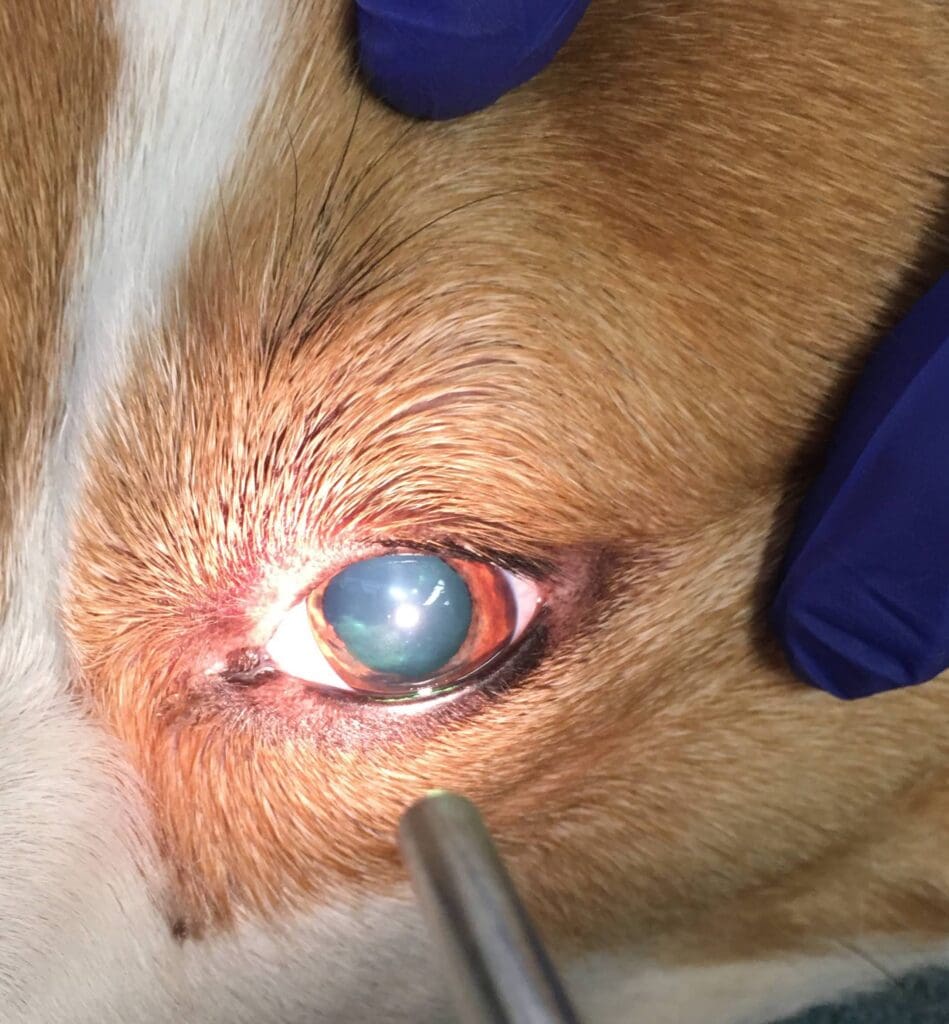
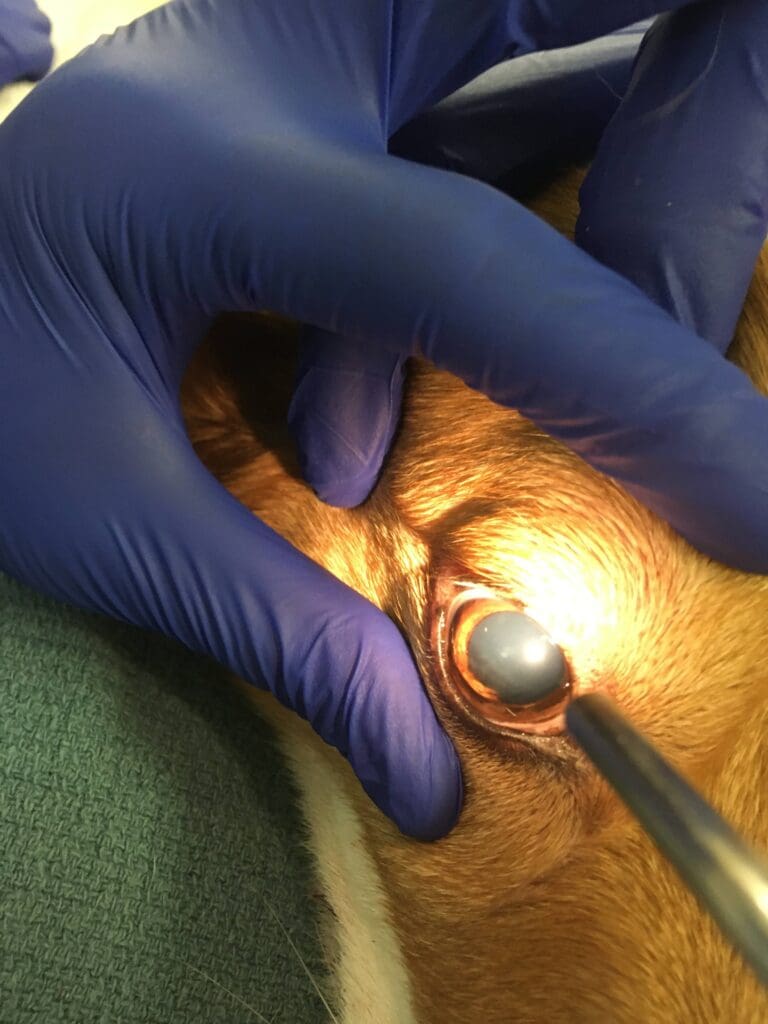
The owners elected humane euthanasia due to a poor prognosis. The necropsy revealed intracerebral hemorrhage and evidence of widespread multifocal bleeding explaining the patient’s clinical signs.
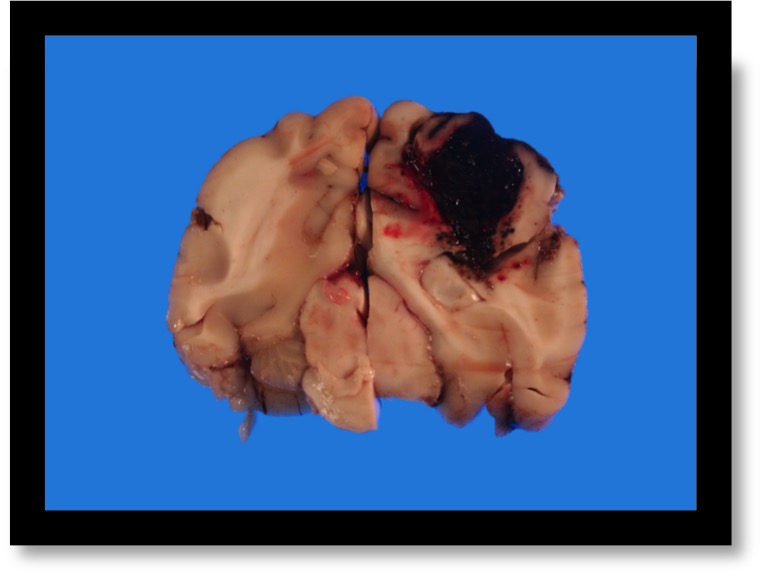
The question I will attempt to answer in this post is whether hyperosmolar therapy improves outcome in humans, dogs or cats with spontaneous intracerebral hemorrhage and signs of intracranial hypertension.
Increased intracranial pressure (ICP) due to intracerebral hemorrhage (ICH) can result from the hematoma itself and from mass effect due to surrounding edema, and may contribute to brain injury and neurologic deterioration (Rordorf et al, 2020). Basic measures of managing elevated ICP include:
- Avoidance of seizures and hyperthermia as they will increase metabolic demands of brain cells;
- Elevation of the head to 30 degrees;
- Avoidance of constrictive central line dressings;
- Use of normal saline initially for maintenance and replacement fluids and avoidance of hypotonic fluids;
- Maintenance of normocapnia and adequate oxygenation;
Osmotic agents (hypertonic saline and mannitol) are commonly used to treat acute ICP elevation or life-threatening mass effect. Hyperosmolar therapy is thought to reduce tissue volume by inducing fluid transfer down osmotic gradients of capillaries and cell membranes (Grande et al, 2012). A few studies have demonstrated efficacy of hyperosmolar therapy for lowering ICP in traumatic brain injury (TBI) (Berger et al, 1995; McGraw et al, 1983) but have failed to show improved clinical outcomes (Guidelines for the Management of Severe Traumatic Brain Injury, 2007). Furthermore, data is sparse regarding its efficacy and ICP-lowering action in spontaneous ICH and post-ischemic edema. In addition, some clinicians have valid concerns with respect to possible exacerbation of intracranial bleeding induced by volume expansion secondary to hyperosmolar agent administration.
Let’s take a look at the available evidence.
The ERICH study was published in 2018 where authors aimed to identify the effect of hyperosmolar therapy (mannitol and/or hypertonic saline) on outcomes after intracerebral hemorrhage (ICH) in people. Treated cases were matched 1:1 to untreated cases by propensity score (combination of age, initial Glasgow Coma Scale, location of the bleeding, etc), sex, and race/ethnicity. They ended up enrolling 2279 cases, of which 304 hyperosmolar-treated cases were matched to 304 untreated cases (without hyperosmolar therapy but otherwise standard treatment similar to the other group). They found that treated cases had worse outcome measured by modified Rankin Scale scores at 3 months compared with untreated subjects (the Modified Rankin Scale (mRS) measures degree of disability/dependence after a stroke). The authors concluded that hyperosmolar therapy was not associated with better 3-month mRS outcomes for ICH cases in the ERICH study. However, this finding likely resulted from greater hyperosmolar therapy use in patients with edema and herniation rather than those agents leading to worse outcomes.
In the study by Wagner et al (Stroke, 2011), the authors investigated the effect of early continuous hypertonic saline infusion on development of perihematomal edema after severe spontaneous supratentorial hemorrhage. Patients with spontaneous lobar and basal ganglia/thalamic bleeding were treated with early (<72 hours) continuous hypertonic saline infusion (3%) to achieve sodium of 145 to 155 mmol/L and osmolality of 310 to 320 mOsmol/kg. Evolution of absolute edema volume and relative edema volume (ratio absolute edema volume/initial hematoma volume) was assessed on repeated cranial CT and compared to historical patients identified on database with hematoma >30 mL. In the treatment group, absolute edema volume was significant smaller between day 8 and day 14 (P=0.04) and relative edema volume was significant smaller between day 2 and day 14 (P=0.02). Intracranial pressure crisis (ICP >20 mm Hg for >20 minutes or new anisocoria) occurred less frequently in the treatment group (12 versus 56; P=0.048). In-hospital mortality was 3 (11.5%) in the hypertonic saline group and 16 (25%) in the control group (P=0.078). Side effects theoretically associated with hypertonic saline including cardiac arrhythmia and acute heart and renal failure occurred in both groups to a similar extent. The authors concluded that early and continuous infusion of hypertonic saline in patients with severe spontaneous intracerebral hemorrhage was feasible and safe.
In the paper by Alam et al. (Neurology, 2017), the authors aimed to evaluate the use of hyperosmolar therapy in patients with larger hematomas who might benefit from treatment targeting mass effect and edema. It was a retrospective study of patients with primary ICH. Patients were divided into those who received standard care or hyperosmolar therapy (mannitol or hypertonic saline). Primary outcome measure was good outcome as measured by modified Rankin Scale Score at discharge. Of 103 patients, 51 received standard care and 52 received hyperosmolar therapy. There were no significant differences in important baseline characteristics: GCS (median 6 in standard care vs 4 in hyperosmolar therapy, P=0.065), presence of intraventricular hemorrhage, location and median hematoma volume. In the standard care group 26.92% patients vs. 11.76% patients in the hyperosmolar therapy underwent hemicraniectomy (P=0.0518). Good outcome at discharge was seen in 13.5% of standard care vs. 2.0% of hyperosmolar treated patients (unadjusted OR 0.129, 95% CI 0.015–1.086, p=0.0599). After adjusting for platelet transfusion, the results were similar (adjusted OR 0.129, 95% CI 0.015–1.094, p=0.0604). The authors concluded that their results were consistent with prior investigations and suggested that hyperosmolar therapy may not benefit patients with large ICH.
A randomized control study on the use of mannitol in spontaneous intracerebral hemorrhage was published by Misra et al. in 2005. In that study, the authors included 128 CT proven supratentorial ICH patients within 6 days of ictus. They were randomized into study and control groups. The study group received rather small doses of mannitol 20%, 100 ml every 4 hours for 5 days, tapered in the next 2 days. This dose equals to 0.3 g/kg, which is lower than we normally use in veterinary patients (0.5-1.5 g/kg). The control group received sham infusion. Primary endpoint was 1-month mortality and secondary endpoint functional disability at 3 months. There were 65 patients in study and 63 in control groups. The study and control groups were evenly matched regarding age, Glasgow coma scale (GCS) score, Canadian Neurological Scale (CNS) score, pupillary asymmetry, etc. At 1 month, 16 patients died in each group. The primary and secondary endpoints were not significantly different between the two groups. They concluded that low dose mannitol does not seem to be beneficial in patients with ICH.
Unfortunately, no veterinary papers looking at hyperosmolar therapy in patients with spontaneous intracerebral hemorrhage were found.
THE BOTTOM LINE
As you can see from the mentioned-above studies, available evidence in human medicine is rather scarce, and identified papers were either retrospective or were performed on relatively small population of patients with treatment protocols not commonly used in veterinary medicine. In my opinion, it is fair to conclude that we still don’t know if hyperosmolar agents make a difference in outcome of patients similar to the case I described in the beginning of this post. If I am presented with a patient that shows signs of progressive intracranial hypertension and possible brain herniation secondary to a spontaneous intracerebral hemorrhage, I will likely use hyperosmolar therapy as a last-ditch effort.
References
- Rordorf et al. Spontaneous intracerebral hemorrhage: Treatment and prognosis. UpToDate, 2020.
- Grande et al. Osmotherapy in Brain Edema. J Neurosurg Anesthesiol, 2012.
- Berger et al. Reduction of post-traumatic intracranial hypertension by hypertonic/hyperoncotic saline/dextran and hypertonic mannitol. Neurosurgery, 1995.
- McGraw et al. Effect of mannitol on increased intracranial pressure. Neurosurgery, 1983.
- Guidelines for the Management of Severe Traumatic Brain Injury. J Neurotrauma (3rd), 2007.
- Shah et al. Effect of hyperosmolar therapy on outcome following spontaneous intracerebral haemorrhage: ERICH Study. J Stroke Cerebrovasc Dis, 2018.
- Wagner et al. Effects of continuous hypertonic saline infusion on perihemorrhagic edema evolution. Stroke, 2011.
- Alam et al. Hyperosmolar therapy is not associated with improved outcomes in patients with large Intracerebral Hemorrhage. Neurology, 2017.
- Misra et al. Mannitol in intracerebral hemorrhage: A randomized controlled study. Journal of neurological sciences, 2005.

Igor, you’re such a baller! These are awesome! I just binge read them all!
Thank you, mentor!
I don’t think the title of your article matches the content lol. Just kidding, mainly because I had some doubts after reading the article.
Your article helped me a lot, is there any more related content? Thanks!
Your article helped me a lot, is there any more related content? Thanks!
Can you be more specific about the content of your article? After reading it, I still have some doubts. Hope you can help me.