Those of you who have ever participated in canine blood donation might have encountered a situation when the blood flow through a sampling line has significantly slowed down or even completely stopped, and despite your best efforts, you had to abort the mission. As a result, the blood bag may end up being half full and contain only 200-250 ml of blood. Since the majority of commercially available donor bags contain 63-70 ml of citrate-based anticoagulant, they are designed to be filled up with 400-450 ml of donor blood to maintain a 1:7 – 1:9 citrate-to-blood ratio. If not, the citrate-to-blood ratio goes up, which creates a risk for citrate toxicity during a blood transfusion. In this situation, a clinician or technician may elect to waste this blood product and send this donor home since collecting another full bag of blood will not be safe for the donor animal. On the other hand, there is an extreme shortage of blood products in veterinary medicine, and, if possible, any waste should be avoided. In this blog post, I will give you a tool that may help you decide on whether a blood product with a high citrate-to-blood ratio should be discarded or safely given to a prospective recipient.
Stored blood is anticoagulated using citrate (~ 3 g in a 450-ml whole blood bag), which chelates calcium. In a healthy adult human, the liver metabolizes 3 g of citrate in 5 min (Li et al. 2015). Infusion rates greater than 1 unit of RBC/5 min, or liver dysfunction, drive citrate elevation and lower plasma ionized calcium (Li et al. 2015; Lima et al. 2014). According to some human medicine literature, citrate toxicity may be prevented in these cases if the transfusion rate is kept below 1 ml/kg/min when using standard blood products with a normal citrate-to-blood ratio (Li et al. 2015).
Citrate intoxication is a frequent complication after massive blood transfusions (Refaai et al. 2013). Besides hypocalcemia, it may lead to metabolic acidosis or alkalosis (the latter is more common). Metabolic alkalosis occurs after citrate gets converted into bicarbonate in the liver. In case of severe liver dysfunction or during early phases of citrate administration, citrate may cause high anion gap metabolic acidosis similar to lactic acidosis.
Severe hypocalcemia is still the most dangerous complication of citrate toxicity, and it may lead to acute vomiting/nausea, reddening of the pinna, tachycardia, ECG abnormalities (see below), arterial hypotension, seizures, tetany and even sudden death (Fukuda et al. 2006).
| ECG abnormalities in patients with hypocalcemia |
| Sinus tachycardia |
| Corrected QT prolongation |
| Dysrhythmias are uncommon, however atrial fibrillation has been reported |
| Torsades de pointes |
(https://litfl.com/hypocalcaemia-ecg-library/)
The following factors must be considered when determining the risk of citrate toxicity development in an individual patient:
- A citrate-to-blood ratio in the blood product
- Rate of blood product administration
- Patient’s body weight
- Liver function
- Presence of hypothermia and/or cardiovascular dysfunction (these conditions will slow down citrate metabolism)
- Presence of pre-existing hypocalcemia, metabolic or respiratory alkalosis
Unfortunately, human guidelines and recommendations cannot be directly applied to veterinary patients, especially when dealing with the situation when the citrate-to-blood ratio is altered.
To overcome this challenge, I have created a simple Excel-based calculator that will allow veterinarians and veterinary technicians to assess the risk of possible citrate toxicity in cases when there is an increased citrate-to-blood ratio in the donor blood product.
Fukuda et al. published an experimental research where it was demonstrated that the tolerable infusion rate of citrate in conscious dogs is 0.33 mmol/kg/h (~64 mg/kg/hr), which was based on clinical signs, ECG and ionized calcium monitoring (See Figure 2). This cut-off was used in the calculator.
In addition, this calculator is designed for standard 450-ml donor bags that contain 63 ml of anticoagulant with 3% citrate (e.g. CPDA-1, CPD, CP2D, ACD-A).
The following formula was used to calculate the citrate rate of administration in mmol/kg/hour based on the total volume of blood and anticoagulant in the bag, recipient’s body weight, anticipated transfusion volume and anticipated time of transfusion:
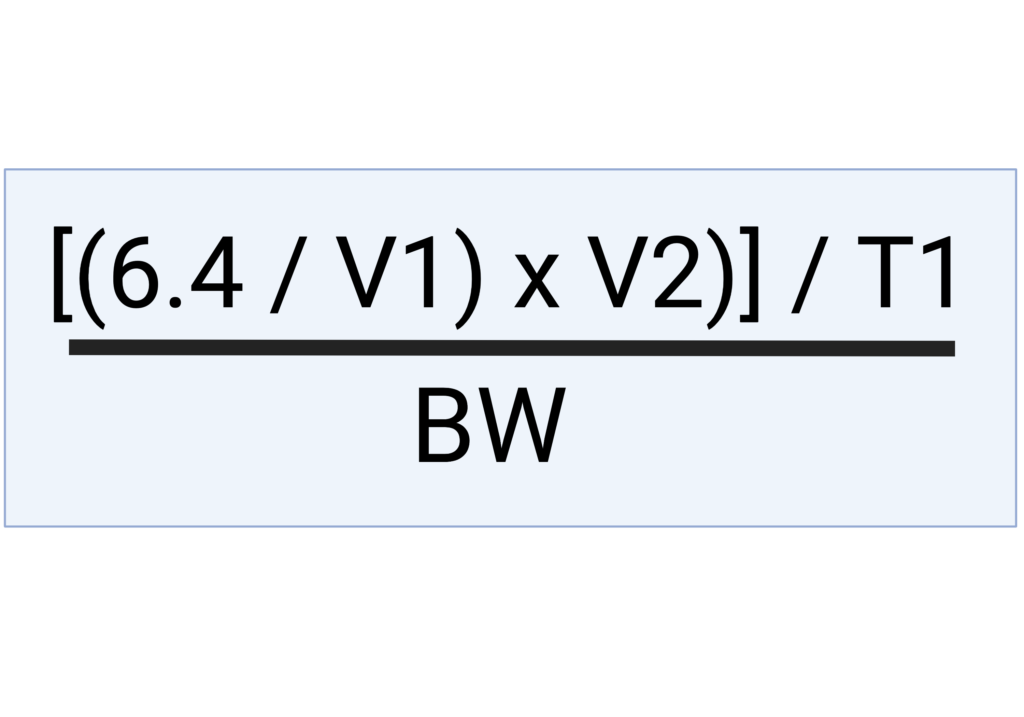
V2 = anticipated transfusion volume
T1 = anticipated time of transfusion
BW = body weight in kg
6.4 = amount of citrate (mmol) in 63 ml of citrate-containing solution such as CPDA-1. The math that resulted in this specific number is demonstrated on the second tab of the Excel calculator.
Here is the example of how this calculator can be used in real practice:
Let’s say you are collecting blood from a canine donor, but you obtained only 190 ml of fresh whole blood because the dog moved and the needle came out of the jugular vein. Your donor blood bag contains 190 ml of fresh whole blood + 63 ml of CPDA-1 anticoagulant resulting in a total volume of 253 ml (blood + anticoagulant). The potential recipient weighs 12 kg. You want to administer all 253 ml (~21 ml/kg) of whole blood over 2 hours since the recipient is not very stable and cannot wait longer.
Now, the question you might have is whether the available donor blood with a high citrate-to-blood ratio (1:3 instead of 1:7 – 1:9) is going to be safe to be administered to this particular recipient over 2 hours?
To solve this problem using the Citrate Toxicity Calculator, you are going to enter patient’s information and the total volume of blood with anticoagulant under the “Enter information below for patient” field (see below, Figure 3), and the calculator will convert this information into the rate of citrate administration in mmol/kg/hour which can be interpreted based on the previously published research (see Figure 3). As you can see, the rate of citrate administration will end up being 0.26 mmol/kg/hour which falls into a safe range (green) based on the Fukuda et al. paper.
The citrate toxicity calculator for dogs can be downloaded here:

The EDUCATIONAL VIDEO CAN BE WATCHED HERE:
References:
- Li K, Xu Y. Citrate metabolism in blood transfusions and its relationship due to metabolic alkalosis and respiratory acidosis. Int J Clin Exp Med. 2015 Apr 15;8(4):6578-84. PMID: 26131288; PMCID: PMC4483798.
- Lima SK, Begum M, Gupta AK, Aziz L, Mitra S. Management of Massive Blood Transfusion-a case study. Pulse. 2014;5:39–43.
- Refaai MA, Blumberg N. The transfusion dilemma-Weighing the known and newly proposed risks of blood transfusions against the uncertain benefits. Best Pract Res Clin Anaesthesiol. 2013;27:17–35.
- Fukuda T, Toyoshima S, Nakashima Y, Koshitani O, Kawaguchi Y, Momii A. Tolerable infusion rate of citrate based on clinical signs and the electrocardiogram in conscious dogs. Clin Nutr. 2006 Dec;25(6):984-93. doi: 10.1016/j.clnu.2006.01.011. Epub 2006 May 15. PMID: 16698131.
- Greening DW, Glenister KM, Sparrow RL, Simpson RJ. International blood collection and storage: clinical use of blood products. J Proteomics. 2010 Jan 3;73(3):386-95. doi: 10.1016/j.jprot.2009.07.011. Epub 2009 Aug 4. PMID: 19664733.
